Every week, there’s a handful of potential custom medical equipment customers inquiring whether PEKO can be the right contract manufacturing company to build their equipment. Last week was no different. A group of seasoned experts from a large bioscience company was visiting us for factory acceptance testing and to learn a little more about the company.

While that was all interesting enough from the perspective of a contact manufacturer, what we found most interesting were the comments from these experts that highlighted some of the industry aggravations they are dealing with daily. What else was interesting is that not only was I hearing the same types of frustrations from these industry experts at a huge corporation as I do from new product teams at medical startups, but how their problems were magnified even more because they were from a large corporation.
For context, my visitors were from a worldwide bioscience company concerned with expanding their footprint into a specialized market. They were part of a highly regarded new product development team bringing a new machine line to market very quickly. The team consisted of the Sourcing Manager, the Quality Manager, and the Head of Product Development.
It was fascinating how the members of the team with the same goal all had wildly different problems that were holding up their portion of the project. I’m going to bring some of these to light today and provide some commentary.
1. How Do You Deal with Spikes in Demand?
Within 45 minutes of the meeting, the Sourcing Manager was asking how we handle demand spikes. Now it’s easy to say what everyone else says… that “we’re perfectly positioned and have all the labor and processes prepared to deal with it.” But the question I wanted to know was “Why is he really asking about this?”
As it turns out, he previously had been working with a supplier in a completely different commodity. They got slammed with a demand spike of 300% volume increase, but weren’t notified until the order came through. Now the production line is down and both parties are suffering (and I think a little peeved at one another). No one is sure who to blame, not that it matters, but it makes one ask, “What could have been done better? What’s the solution here?”

In the case of custom medical equipment, there’s not always a forecast on which you can rely. But it stands to reason that someone had an inkling that there would be an order of four times the original demand. There must have been some executives or a Program Manager that were negotiating the contract. Even so much as a week’s notice could have given the internal and outsource team an idea of what was coming so they could get a head start. If it was known long before that, then there’s an even larger communication problem.
You see, when dealing with new products or even production equipment, the OEM and Contract Manufacturer benefit from keeping open lines of communication. The CM acts as extension of their manufacturing operations, so he or she must be notified of the sales pipeline so preparation can occur.
In my opinion, if communication was the issue, this could’ve been resolved by either party. A periodic review should be scheduled by either the customer or the CM and the sales forecast must be discussed. Proper risk mitigation techniques can be put in place so that when the demand spikes, there is an action plan to deal with it. If the spike never does hit, then you stick the plan in the drawer for another day.
2. What Is Your Factory Acceptance Testing (FAT) Procedure?
With any new product, but especially for custom medical equipment for the bioscience industry, everyone should be thinking about “What’s good? How do we define ‘good’?” At the end of the day, a machine or piece of complicated equipment must leave the factory for its next destination working properly. But this is much easier said than done.
Factory acceptance testing, or FAT, is the procedure used to ensure both parties agree on what is considered “good” for the product to leave the factory floor. In our experience, most companies do not properly define criteria for factory acceptance testing. According to Control Design, “…there is no set checklist during a FAT; it can consist of a variety of inspection points and tests per the request of the customer, based on your requirements and unique equipment specifications.” Furthermore, what if it doesn’t pass the FAT? What happens then? Does the company have a procedure for that?

Quality engineer at PEKO performing an in-depth quality check, including Factory Acceptance Testing (FAT), on a custom medical equipment build.
The customer’s Quality Manager spent a long time on these questions for two very good reason. Of course, the first is that he wanted to make sure his product was being produced correctly. But secondly and even more interesting, his company did not have a standardized FAT procedure for this type of equipment. That’s right, a multi-billion-dollar company with thousands of employees did not have a written test procedure.
But if you think about it, it is no fault of their own. With custom medical equipment, there needs to be a customized FAT. Of course, there are templates and precedents, but since we’re talking about new-to-market equipment, then we are requiring a fully customized FAT to reflect the intricacies of the machine. In this case, we had developed an FAT for the customers equipment but developing it and implementing it are two very different things, which is exactly why he was concerned and why he was on our factory floor that day.
3. How Will My Project Be Transitioned From R&D to Production?
The Ph.D. who served as the Head of Product Development (there’s almost always a Ph.D. in those R&D departments!) knew what a lot of R&D folks know. His team had busted their butts to design this machine and figure out the science behind it, but it was far from being optimized for manufacturing and production.
Typically, the early life cycles of products, especially the prototype and alpha builds, are a collage of parts sourced from wherever they could get them fast. Features and pricing can be overlooked at the time, they just need to get the science part proved out and the machinery functioning.

The documentation wasn’t up to snuff, in fact it was barely existent, and hardly any DFMA had been done. This was the main reason he was so concerned with our plan to move the project from the pilot to production phases. This is a mix of design touch-up and process optimization. The other reason he was asking was because, like a lot of customers I have, they need a contract manufacturer who could handle all of these tasks.
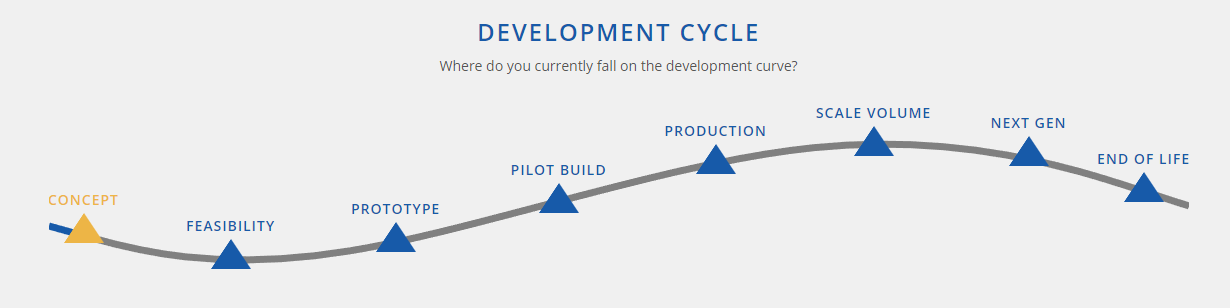
Over the years, people in his very position realize that there is a massive skill gap between prototype and production, so using a contract manufacturing company that can cover the entire product lifecycle is very important to the ultimate success of the commercialization effort.
The Take-Away:
 All told, it was a good day of vigorous manufacturing discussion. Our teams worked hard to finalize the machine, and everyone left happy. The reason I bring these issues to the forefront is that every day I talk to customers with similar problems without good recourse to solve them.
All told, it was a good day of vigorous manufacturing discussion. Our teams worked hard to finalize the machine, and everyone left happy. The reason I bring these issues to the forefront is that every day I talk to customers with similar problems without good recourse to solve them.
If this sounds like you, feel free to reach out to our team for some help. PEKO has been providing full-service medical contract manufacturing support like this for over 55 years and counting. For more guidance, download our Medical Contract Manufacturing Guide to see where you land in the product development curve. In this guide, you’ll also learn what types of issues you should be looking out for to prepare yourself for production and why you should consider the possibility of outsourcing your custom medical equipment build to a contract manufacturer.








